More in News
-


News
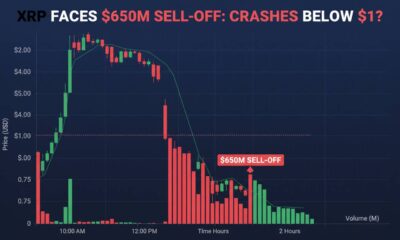
XRP Faces $650M Sell-Off: Will Price Crash Below $1?
XRP is under mounting pressure as on-chain data reveals a staggering $652 million worth of tokens...
-


News
BTC’s Monday Rally Fueled by Short Squeeze, Not Real Demand
Introduction Bitcoin surged nearly 5% on Monday, climbing above $69,000, but the move appears driven by...
-


News
Bitcoin May Have Already Bottomed Against Gold: Analyst Predicts March Recovery
Bitcoin’s relative weakness against gold may finally be nearing its nadir, with some analysts suggesting that...
-


News
$572M in Token Unlocks This Week – Brace for Major Selling Pressure
Crypto markets are entering a potentially turbulent phase as over $572 million worth of tokens are...
-


News
12 EU Banking Giants Unite: Launching Euro Stablecoin in 2026
A powerful consortium of 12 leading European banks has formed Qivalis, a Netherlands-based entity, to issue...
-


News
Bitmine Drops $98M on Ethereum, Now Holds $10B in Digital Assets
Bitmine Immersion Technologies (NYSE: BMNR) recently deepened its Ethereum accumulation, deploying $98 million to acquire 51,162...
-


News
Exchange Files SEC Proposal for Nasdaq-100 Prediction Market
Nasdaq Inc. submitted a formal proposal to the U.S. Securities and Exchange Commission (SEC) on March...
-


News
Bitcoin Approaches 20 Million Coins Mined – Only 1 Million Left to Discover
As of early 2026, Bitcoin is nearing a pivotal milestone: approximately 19.94 million coins have been...
-


News
Crypto Hacks Plunge 69% in February: Lowest Losses in a Year
In February 2026, the cryptocurrency sector experienced a dramatic decline in hack-related losses, with total damages...
-


News
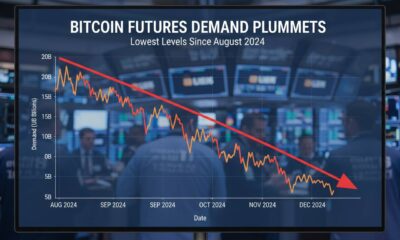
Bitcoin Futures Demand Plummets to Lowest Levels Since August 2024
Introduction Bitcoin futures demand has collapsed to its lowest point since August 2024, signaling a sharp...
-


News
Buterin Dumps More ETH Than Planned – Total Sales Reach $38M
Ethereum co-founder Vitalik Buterin has exceeded his previously announced ETH sell-off plan, offloading significantly more than...
-


News
SpaceX Eyes $1.75T IPO & $545M Bitcoin Stash: What It Means
SpaceX is preparing for what could become the largest IPO in history, targeting a valuation exceeding...
-


News
HyperLiquid: Retail’s Bear Market Playground as HYPE Surges
In the depths of a broader crypto bear market, Hyperliquid’s native token, HYPE, has emerged as...
-


News
Battle for Bitcoin’s Soul: First BIP-110 Block Mined Amid Heated Debate
In a striking development within Bitcoin’s governance landscape, the first block signaling support for BIP‑110 was...
-


News
Vitalik Unveils Anti-Centralization Plan to Combat Toxic MEV on Ethereum
Ethereum co-founder Vitalik Buterin has introduced a fresh set of proposals aimed at curbing centralization in...